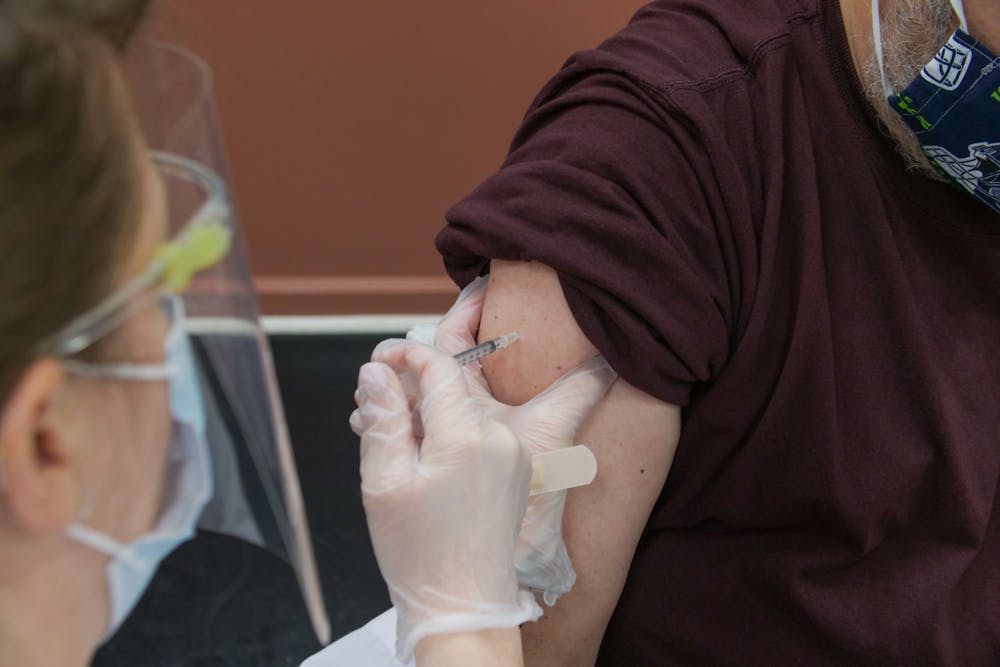
UB will postpone plans to open an on-campus vaccination site after federal authorities called for an immediate nationwide pause on the Johnson & Johnson vaccine due to concerns of rare but severe blood clots.
“In coordination with SUNY and following a recommendation from the Food and Drug Administration and the Centers for Disease Control and Prevention, the University at Buffalo will pause plans to use the Johnson & Johnson single dose coronavirus vaccine at an on-campus vaccination site for students scheduled to open on Thursday in the Student Union,” the university wrote in a statement Tuesday.
University leaders say more information will be communicated with students over the coming days. The J&J vaccine is being paused nationwide “out of an abundance of caution” after six individuals — out of the more than six million who have gotten the one-dose vaccine — developed blood clots.
“SUNY is following the recommendation of the U.S. Food and Drug Administration, Centers for Disease Control and Prevention and the New York State Department of Health to immediately pause, out of an abundance of caution, administering the Johnson & Johnson one-shot vaccine,” SUNY Chancellor Jim Malatras said in a statement.
“We are working with New York State to locate and receive alternative COVID-19 vaccines for our students. We urge all students with appointments for the Johnson & Johnson vaccine to contact their campus or vaccination site because alternatives have already been found in some instances.”
The CDC and FDA issued its recommendations Tuesday after six women — all between the ages of 18 and 48 — developed blood clot symptoms six to 13 days after they received the J&J vaccine. The CDC plans on holding a meeting of the Advisory Committee on Immunization Practices Wednesday to discuss these cases and their potential significance.
In recent days, SUNY provided UB with 200 doses of the J&J vaccine. The J&J vaccine is 72% effective against COVID-19 in the U.S. — and 57% effective in South Africa, which predominantly has a different variant of the virus — and can be delivered in one dose, according to researchers.
UB planned on launching a testing site in the Student Union on Thursday, but those plans were halted due to Tuesday’s CDC and FDA recommendations.
“While we must not slow down the process of protecting our students from the COVID-19 virus, we must do all we can to ensure their safety and health every step of the way,” according to the SUNY statement. “We will keep our campus communities informed as more information becomes available.”
The news desk can be reached at news@ubspectrum.com

Justin Weiss is The Spectrum's managing editor. In his free time, he can be found hiking, playing baseball or throwing things at his TV when his sports teams aren't winning. His words have appeared in Elite Sports New York and the Long Island Herald. He can be found on Twitter @Jwmlb1.